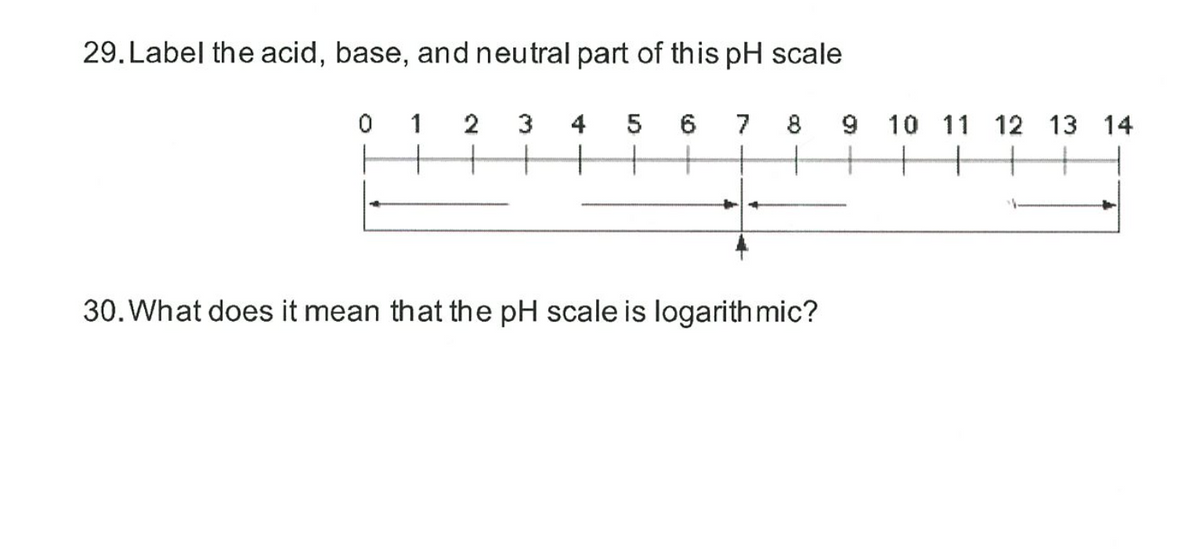
44 label ph scale
pH and Water | U.S. Geological Survey - USGS.gov pH is a measure of how acidic/basic water is. The range goes from 0 to 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that ... Acids, Bases, & the pH Scale - Science Buddies In order to deal with these large numbers more easily, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14.
What is the pH scale and what does it measure? - BBC Bitesize The pH scale shows how acidic a substance is. It can be measured using a pH meter which gives a numerical value. The pH scale ranges from 0 (very acidic ) through 7 ( neutral ) to 14 (very...
Label ph scale
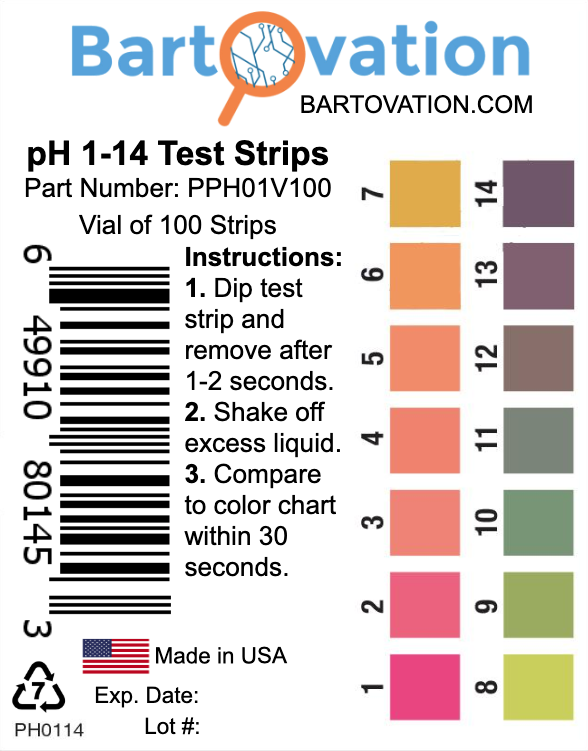
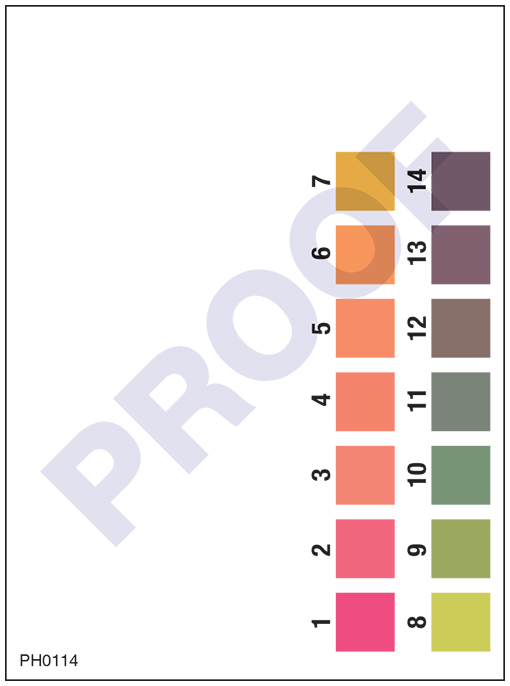
Draw a pH scale and label acids, bases and salts on the scale. Ab Padhai karo bina ads ke. Khareedo DN Pro and dekho sari videos bina kisi ad ki rukaavat ke! Login if already purchased ... pH, pOH, and the pH scale (article) | Khan Academy Hand holding wet pH paper with four stripes (from left to right): orange, green-brown, yellow, and red-orange. The paper is held up for comparison against a reference chart of pH values and colors. The wet paper matches the pH 7 on the reference. The pH Scale - Chemistry LibreTexts The pH scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. For example, a pH of 3 is ten times more acidic than a pH of 4. Likewise, a pH of 3 is one hundred times more acidic than a pH of 5. Similarly a pH of 11 is ten times more basic than a pH of 10.
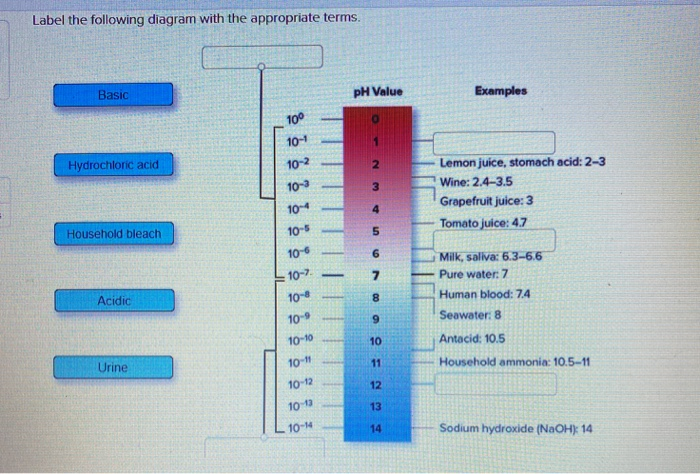
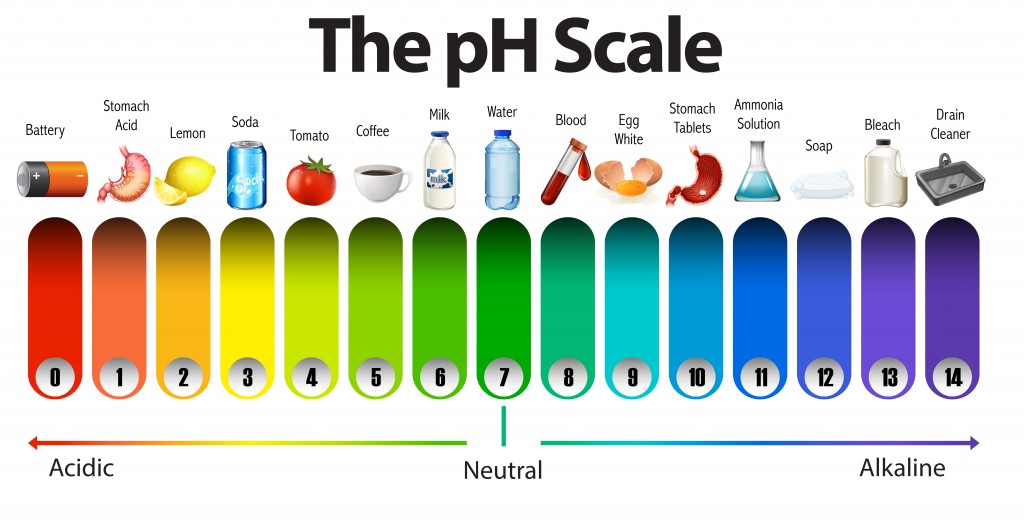
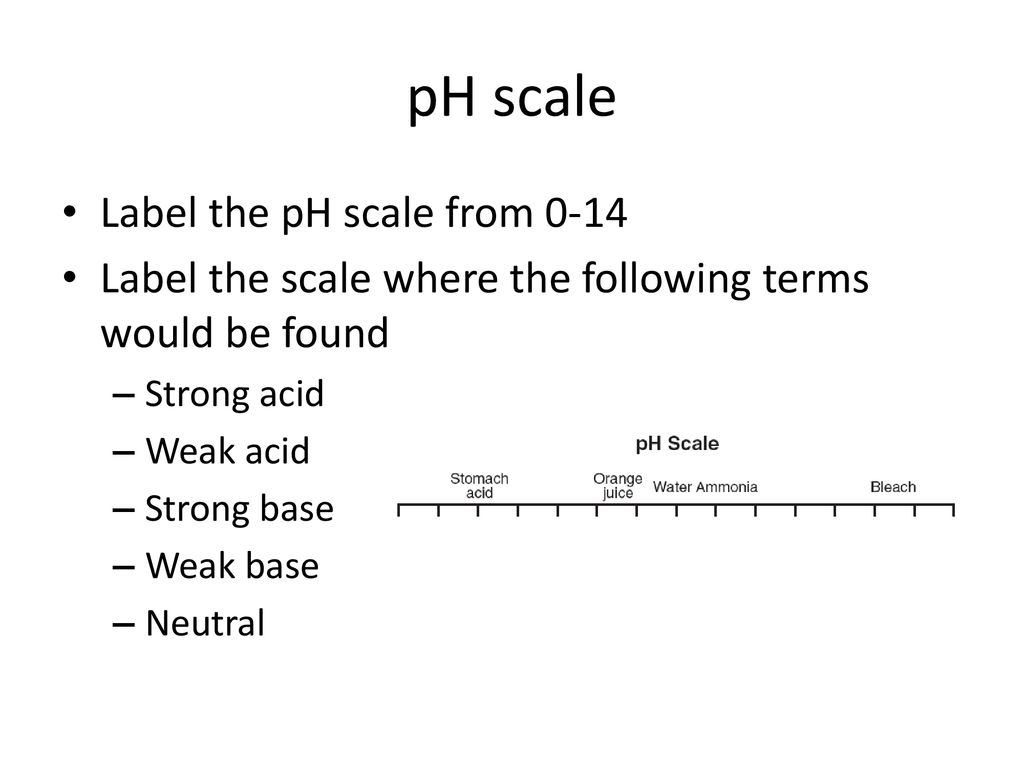
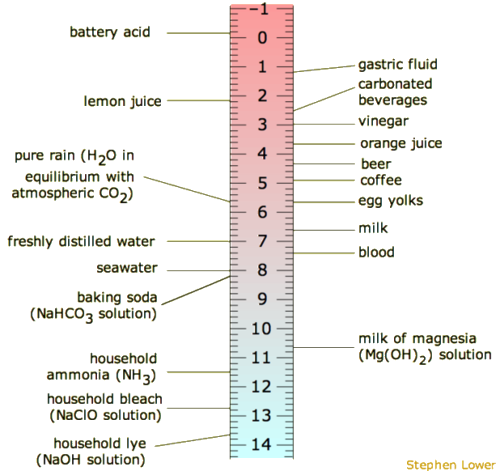
Label ph scale. The pH scale - Acids, bases and salts - (CCEA) - BBC The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH... pH Scale - PhET Interactive Simulations pH Scale - PhET Interactive Simulations 14.9: The pH and pOH Scales- Ways to Express Acidity and Basicity pH is usually (but not always) between 0 and 14. Knowing the dependence of pH on [H3O +], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the pH scale. The pH scale with some common examples - NOAA Pacific Marine ... The pH scale with some common examples. Home. Research. Ocean Acidification. Ocean Carbon Uptake. Ocean Carbon Storage. Coastal Carbon Dynamics. Observations. Volunteer Observing Ships (VOS)
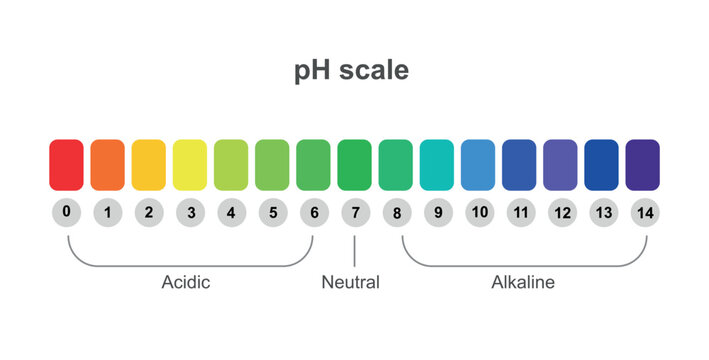
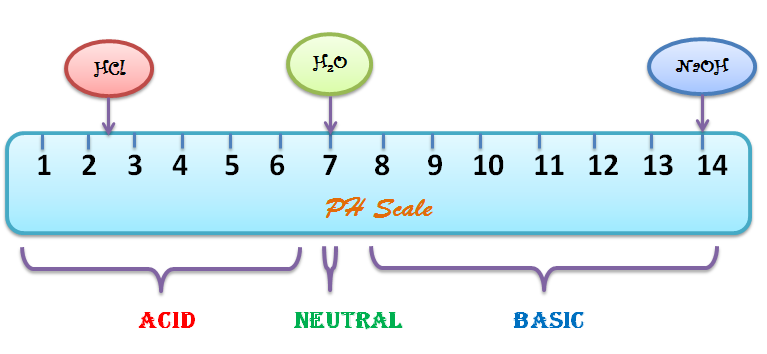
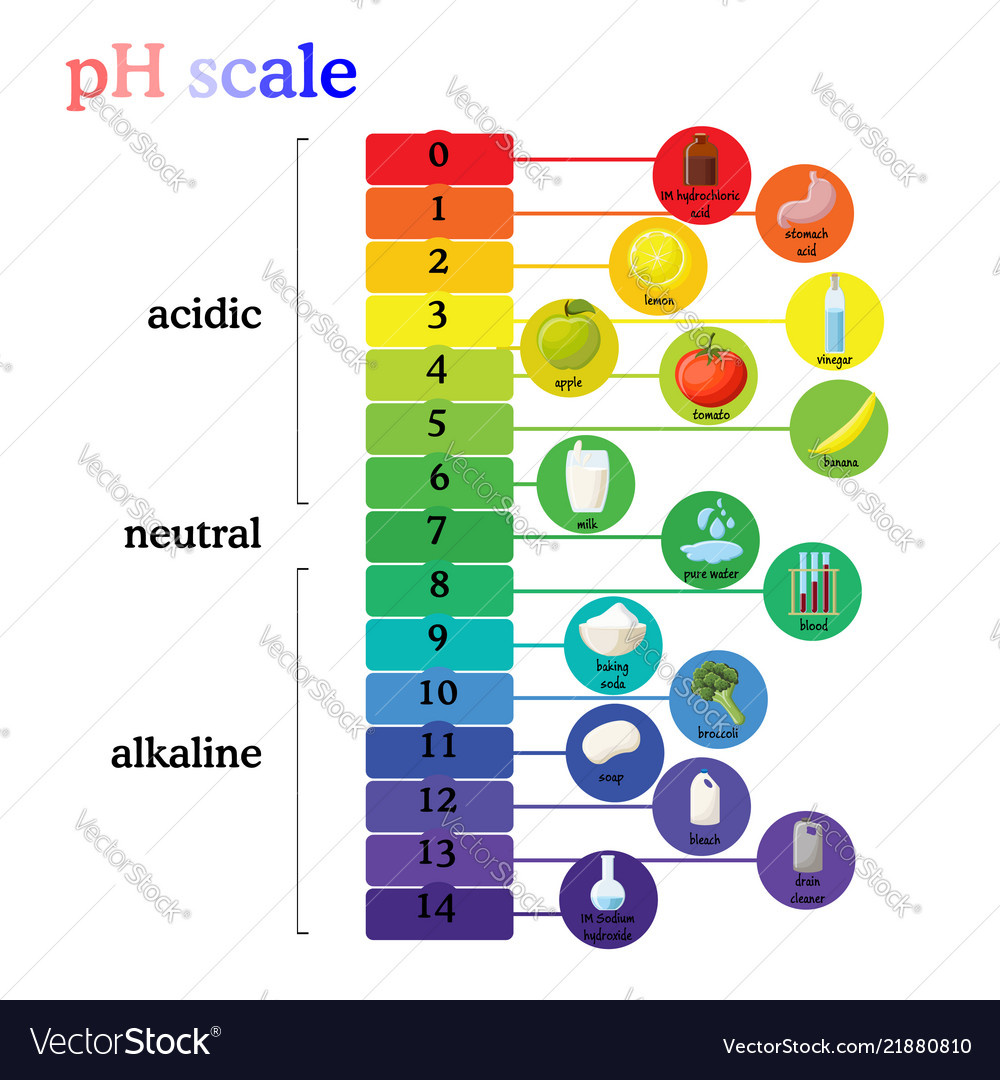
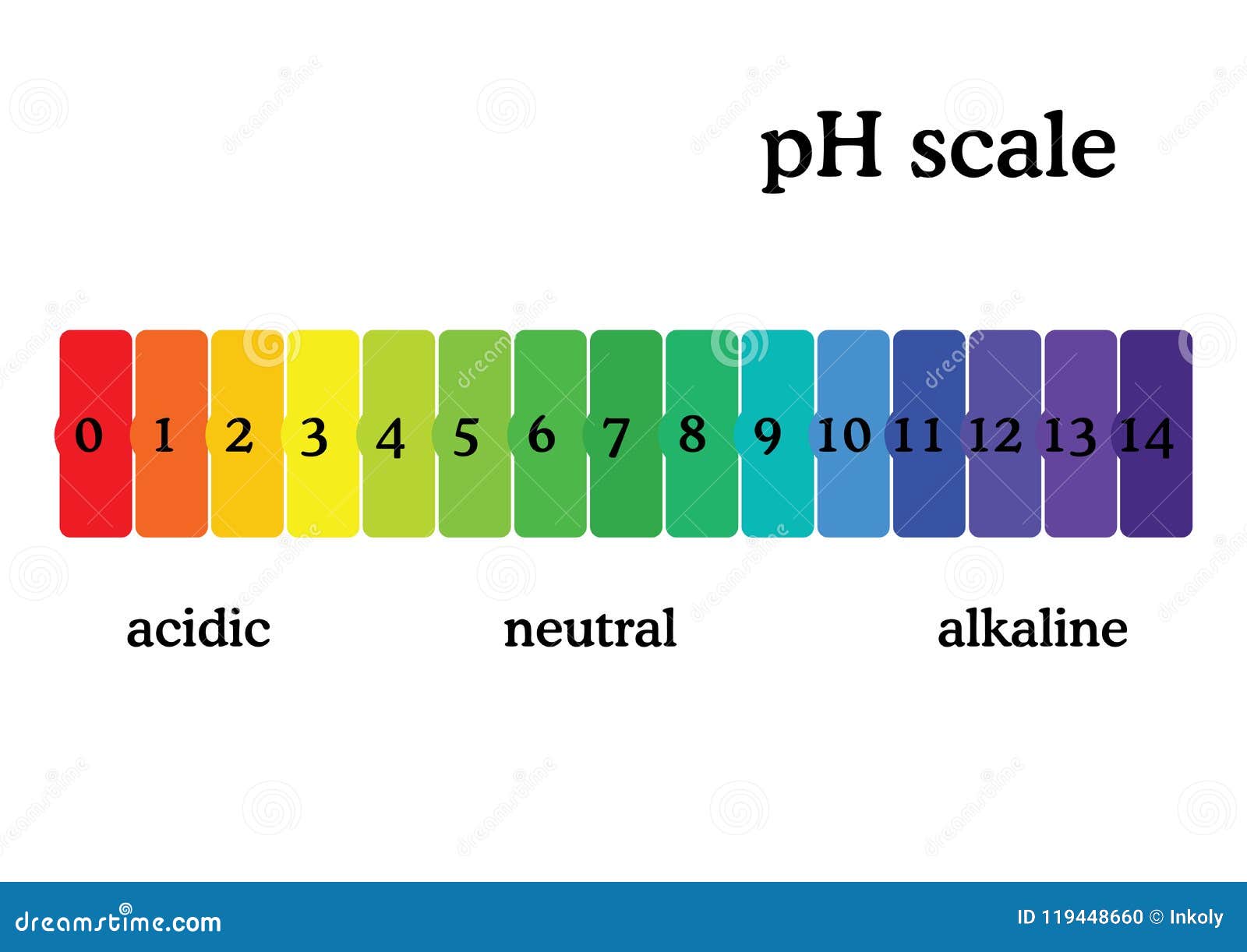
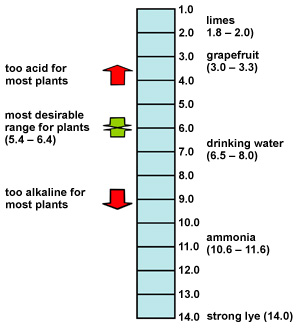
Draw pH scale and label acids alkalis and neutral. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to ... Label the pH scale Diagram - Quizlet Start studying Label the pH scale. Learn vocabulary, terms, and more with flashcards, games, and other study tools. pH Scale | U.S. Geological Survey - USGS.gov pH is a measure of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Interactive Notebook: Acids and Bases pH Scale - Pinterest ... with specific pH values, then draw and label those substances in the worksheet. Students then color the given pH scale reflected in pH indicator pap...
7.6: The pH and pOH Scales - Chemistry LibreTexts Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5; pure water, pH = 7; wine, pH = 3.0; ... pH is a logarithmic scale. A solution that has a pH of 1.0 has 10 times the [H +] as a solution with a pH of 2.0, which in turn has 10 times the ... PDF pH Scale Activity - birdvilleschools.net pH Scale Activity 1. On the construction paper, NEATLY draw a pH scale. 2. Scale the line from 0 to 14 with a mark for each number. 3. Cut out words & paste the labels in correct areas of pH scale. Weak Acid Strong Acid Strong Base Weak Base Neutral . 4. Color & Label the pH on the picture. Cut out & paste in the correct sections of the pH scale. Solved Label the pH scale below, indicating the acidity of a - Chegg Label the pH scale below, indicating the acidity of a solution in the given regions. Indicate the color of phenolphthalein within the pH ranges shown below. Why is phenolphthalein an appropriate indicator for a weak acid-strong base titration? A. The pH Scale - GitHub Pages This is known as the pH scale The range of values from 0 to 14 that describes the acidity or basicity of a ... Example 12. Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5; pure water, pH = 7; wine, pH = 3.0; Solution. With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia ...
Draw neat and labeled diagram of pH scale? - Toppr Draw neat and labeled diagram of pH scale? Medium Solution Verified by Toppr The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is used to rank solutions in terms of acidity or basicity (alkalinity). Since the scale is based on pH values, it is logarithmic, meaning that a change of 1 pH unit corresponds to a ten-fold change in H + ^+ + start superscript, plus, end superscript ion concentration. The pH scale is often said to range from 0 to 14, and most ...
Question Video: Identifying the Correctly Labeled pH Scale - Nagwa The pH scale, with values generally falling between zero and 14, measures the acidity or basicity of a substance. Substances with a pH below ...
6.3: The pH Scale - Mathematics LibreTexts This is known as the pH scale. You can use pH to make a quick determination of whether a given water based solution is acidic, basic, or neutral. ... Example \(\PageIndex{1}\) Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5; pure water, pH = 7; wine, pH = 3.0; Solution. With a pH greater ...
pH Scale | U.S. Geological Survey - USGS.gov The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic.
14.9: The pH and pOH Scales - Chemistry LibreTexts The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral. ... {H_3O^+}] = 10^{-pH} \label{ph1} \] As mentioned above, different calculators work slightly differently—make sure you can do ...
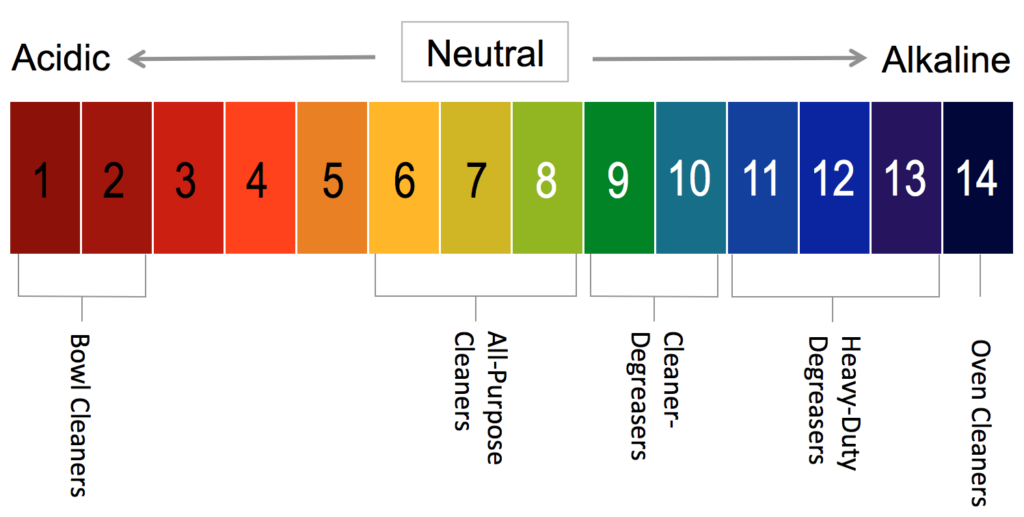
Ph scale with colored labels of environments Vector Image ph scale with colored labels of environments and products. Download a free preview or high-quality Adobe Illustrator (ai), EPS, PDF vectors and high-res ...
10.11: The pH and pOH Scales - Chemistry LibreTexts The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral. ... {H_3O^+}] = 10^{-pH} \label{ph1} \] As mentioned above, different calculators work slightly differently—make sure you can do ...
The pH Scale - Chemistry LibreTexts The pH scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. For example, a pH of 3 is ten times more acidic than a pH of 4. Likewise, a pH of 3 is one hundred times more acidic than a pH of 5. Similarly a pH of 11 is ten times more basic than a pH of 10.
pH, pOH, and the pH scale (article) | Khan Academy Hand holding wet pH paper with four stripes (from left to right): orange, green-brown, yellow, and red-orange. The paper is held up for comparison against a reference chart of pH values and colors. The wet paper matches the pH 7 on the reference.
Draw a pH scale and label acids, bases and salts on the scale. Ab Padhai karo bina ads ke. Khareedo DN Pro and dekho sari videos bina kisi ad ki rukaavat ke! Login if already purchased ...




























Komentar
Posting Komentar